Global Drug of Abuse Testing Market Set as Compliance-Driven Screening Becomes a Core Healthcare and Workplace Standard
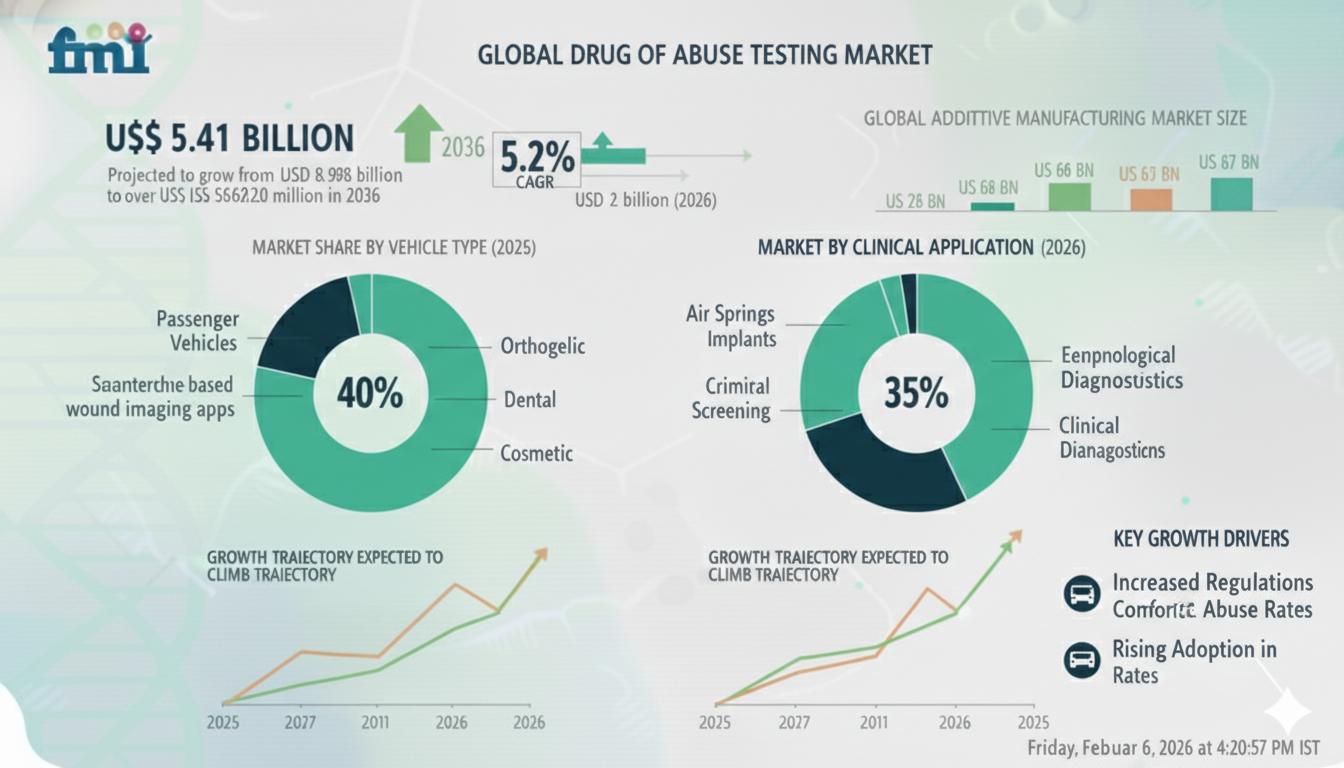
NEWARK, DE / ACCESS Newswire / February 6, 2026 / The global diagnostics and public safety landscape is undergoing a critical transformation as drug screening shifts from discretionary testing to a compliance-critical diagnostic service. Driven by rising substance use, regulatory enforcement, and expanding institutional screening programs, the Drug of Abuse Testing Market valued at USD 5.41 billion in 2026 is projected to reach USD 8.98 billion by 2036, expanding at a steady CAGR of 5.2% over the forecast period.
According to the latest analysis from Future Market Insights (FMI), the market's growth is being fueled by increasing adoption of routine screening across hospitals, diagnostic laboratories, forensic facilities, and workplace environments. The integration of immunoassay-based high-throughput screening with confirmatory chromatography methods such as GC-MS and LC-MS is enabling faster decision-making while preserving analytical accuracy and legal defensibility.
From Optional to Essential: Drug Testing as a Compliance Backbone
Drug of abuse testing is no longer viewed as a reactive or optional procedure. In 2026, it has become a foundational component of institutional compliance, occupational safety, and public health surveillance.
"In regulated healthcare and workplace environments, drug testing is now a compliance-driven necessity rather than a discretionary diagnostic service," notes an FMI analyst. "Organizations are prioritizing structured workflows that combine rapid screening with confirmatory testing to meet regulatory and chain-of-custody standards."
This structural shift is reinforcing tiered testing models, where immunoassay screening serves as the front-line triage tool, followed by chromatography-based confirmation for evidentiary and clinical certainty.
Technology Convergence: Speed Meets Analytical Precision
Breakthroughs in analyzer automation, assay specificity, and rapid testing platforms are reshaping competitive dynamics across laboratories and healthcare systems. The market is increasingly defined by integrated testing ecosystems that support:
High-Volume Immunoassay Screening for rapid throughput
Chromatography-Based Confirmation (GC-MS, LC-MS) for legal and clinical defensibility
Rapid Testing Formats for decentralized and point-of-care environments
This convergence allows laboratories to balance speed, sensitivity, and specificity ensuring both operational efficiency and regulatory compliance.
End-User Demand: Hospitals and Diagnostic Labs at the Core
Diagnostic laboratories remain the most influential end-user segment, handling high volumes of samples from hospitals, law enforcement agencies, and occupational health programs. Hospitals rely on rapid testing for acute clinical decision-making, while forensic laboratories prioritize chromatography-based confirmation for analytical precision.
Workplace and occupational health screening is also emerging as a major growth engine, as employers adopt standardized testing protocols to meet safety and regulatory mandates.
Regional Performance: Emerging Markets Accelerate Adoption
While North America maintains market leadership, growth momentum is accelerating across Asia and Europe:
United States (4.7% CAGR): Supported by mature workplace mandates, forensic infrastructure, and institutional screening programs
China (6.1% CAGR): Driven by public safety initiatives, hospital expansion, and workplace compliance
India (6.9% CAGR): Fastest-growing market, fueled by occupational screening, regulatory expansion, and public health initiatives
Germany (5.0% CAGR): Anchored by protocol-driven healthcare and forensic compliance
United Kingdom (4.3% CAGR): Shaped by public health strategies and workplace testing requirements
Japan (3.8% CAGR): Conservative but steady growth focused on analytical precision and system upgrades
Emerging markets are benefiting from rising hospital capacity, automation upgrades, and expanding institutional testing frameworks.
Key Market Metrics (2026-2036)
Metric |
Details |
|---|---|
Market Value (2026) |
USD 5.41 Billion |
Forecast Value (2036) |
USD 8.98 Billion |
Projected CAGR |
5.2% |
Primary Technique |
Immunoassay Screening |
Key Growth Driver |
Regulatory Compliance & Routine Screening |
Competitive Landscape: Innovation and Automation Take Center Stage
The competitive environment is led by major diagnostics and analytical technology providers that are expanding assay menus, automation capabilities, and integrated workflow platforms. Key players include:
Abbott Laboratories (Alere, Inc.)
F. Hoffmann-La Roche Ltd
Siemens AG (Siemens Healthineers)
Thermo Fisher Scientific, Inc.
Shimadzu Corporation
Drägerwerk AG & Co. KGaA
These companies are investing heavily in automated analyzers, high-specificity reagent systems, and modular chromatography platforms to support scalability, throughput efficiency, and regulatory alignment.
Strategic Developments Strengthening Market Positioning
Recent industry developments are further reinforcing market structure:
August 2025: SCRAM Systems acquired PharmChem, strengthening sweat-based drug testing and monitoring capabilities
June 2024: Align Capital Partners-backed Premier Biotech acquired Desert Tox, expanding certified toxicology laboratory services
These moves highlight the industry's shift toward vertically integrated and compliance-focused testing ecosystems.
The Outlook: From Diagnostic Tool to Regulatory Infrastructure
By 2036, drug of abuse testing is expected to be fully embedded as a core regulatory and healthcare infrastructure component. The market's evolution will be defined by:
Alignment with regulatory mandates
Scalable laboratory automation
Integrated screening and confirmation workflows
Demand for defensible, auditable results
As healthcare systems, employers, and public safety agencies continue to formalize testing protocols, drug of abuse testing is positioned to transition from a supportive diagnostic function to a mission-critical compliance service.
For a complete strategic analysis and detailed segmentation outlook for the Drug of Abuse Testing Market through 2036: https://www.futuremarketinsights.com/reports/drug-of-abuse-testing-market
Related Reports:
Drug Testing Systems Market: https://www.futuremarketinsights.com/reports/drug-testing-systems-market
Sports Drug Testing Market: https://www.futuremarketinsights.com/reports/sports-drug-testing-market
Drug Formulation Market: https://www.futuremarketinsights.com/reports/drug-formulation-market
Road Side Drug Testing Devices Market: https://www.futuremarketinsights.com/reports/road-side-drug-testing-devices-market
Drug-Induced Dyskinesia Market: https://www.futuremarketinsights.com/reports/drug-induced-dyskinesia-market
About Future Market Insights (FMI)
Future Market Insights (FMI) is a leading provider of market intelligence and consulting services, serving clients in over 150 countries. Headquartered in Delaware, USA, with a global delivery center in India and offices in the UK and UAE, FMI delivers actionable insights to businesses across industries including automotive, technology, consumer products, manufacturing, energy, and chemicals.
An ESOMAR-certified research organization, FMI provides custom and syndicated market reports and consulting services, supporting both Fortune 1,000 companies and SMEs. Its team of 300+ experienced analysts ensures credible, data-driven insights to help clients navigate global markets and identify growth opportunities.
For Press & Corporate Inquiries
Rahul Singh
AVP - Marketing and Growth Strategy
Future Market Insights, Inc.
+91 8600020075
For Sales - sales@futuremarketinsights.com
For Media - Rahul.singh@futuremarketinsights.com
For web - https://www.futuremarketinsights.com/
SOURCE: Future Market Insights, Inc.
View the original press release on ACCESS Newswire




